BioDock can store as little as a single cryobox in one of many storage vessels.
Sample storage and disaster recovery services
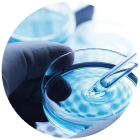
Sample Storage
HTA and HFEA relevant materials (stem cells, embryos, ovaries), research archival inventories, master cell lines and reagent storage.
Bio-Services
Ultilising our laboratory for aliquoting, sample processing, flow cytometry, sterility testing and more.
Disaster recovery
Emergency relocation of samples or vessels in the event of an equipment, supplier or other failure.
Biobanking consultancy
Expert knowledge across the biobanking industry – commercially, logistically and scientifically.

Biological Sample Storage Solutions
An industry leader in the storage of biological materials, with state-of-the-art facilities designed to support hospitals and research institutions in expanding their storage capacity.
Based in the UK, we have stored over 1 million samples from around the world. A fast-paced approach allows institutions to quickly increase their storage capacity in a trusted, accredited facility.
Our tailored and robust disaster recovery services provide a vital resource in event of an emergency.

About Us
As industry leaders and consultants in the storage of biological materials, we offer a unique mix of experience, scalability and security that is second-to-none.
Over 20 years’ of experience in sample storage
On-site 24 hour security
1,000,000 samples stored across 100+ storage vessels

Fully validated equipment
Dedicated specialist courier service
Range of temperature conditions
Quality management system controlled processes

Secure data management systems

Part 11, 21 CFR compliant systems
State-of-the-art facilities
Dual Storage locations available for superior security, and on-site biobanking and genomics laboratories
At BioDock, we heavily invest in our facilities to ensure that our client’s samples are stored optimally.
Frequently Asked Questions
BioDock can store samples in a range of temperatures including: 2-8°C, -20°C, -80°C and -196°C.
BioDock has extensive experience in designing and executing a range of complex service provisions using our cryogenic storage facility and state-of-the-art laboratory. Get in touch with one of our specialists to discuss your requirements.
BioDock is proud to provide exceptionally quick shipment times – often despatching samples within 24 hours of request, using our in-house courier.
What our clients say
We provide a bespoke solution for our clients' unique requirements
Our accreditations